Further Evidence for Cancer as a Cell-Wall-Deficient Mycobacterial Disease
Lysenko AP, Broxmeyer L, Vlasenko VV, Krasochko PA, Lemish AP and Krasnikova EA
Lysenko AP1, Broxmeyer L2*, Vlasenko VV3, Krasochko PA1, Lemish AP1 and Krasnikova EA1
1Institute of an Experimental Veterinary Science n. S.N. Wyshelesski, Belarus
2N.Y. Institute of Medical Research in Bayside, New York, USA
3Podolsk Center of Tuberculosis, Ukraine
- *Corresponding Author:
- Lawrence Broxmeyer, M.D
N.Y. Institute of Medical Research in Bayside,
New York, USA.
Tel: 011-718-229-3694
E-mail: drlawrencebroxmeyermd@alumni.usc.edu
Received date: October 07, 2016; Accepted date: November 03, 2016; Published date: November 14, 2016
Citation: Lysenko AP, Broxmeyer L, Vlasenko VV , et al. Further Evidence for Cancer as a Cell-wall-deficient Mycobacterial Disease. J Mol Path Epidemol. 2016, 1:1.
Abstract
In 2014, Buehring reported that Bovine Leukemic Virus(BLV), a common oncogenic retrovirus of cattle, was present in some humans, primarily localized to the breast epithelium ― the very cell type from which most breast malignancies arise. By 2015, there appeared data (Buehring, 2015) supporting that as many as 37% of human breast cancer cases could be attributable to BLV exposure. But if recent estimates suggest over 83% of U.S. dairy operations are currently positive for BLV, they also show that approximately 68% are positive for cell-wall-deficient Mycobacterium avium subspecies paratuberculosis (MAP). Although tubercular lung infection has been said to cause 11 times the incidence of lung cancer as normal control subjects, it is its cell-wall-deficient (CWD) forms (also called L-forms) that have recently repeatedly been found through genetic analysis and appropriate stains in such cancer tissue ― suggesting that CWD tuberculosis or atypical tuberculosis “is likely to be involved in the occurrence or development of lung carcinoma”. A similar relationship between tubercular L-forms and the genesis of the very breast cancer addressed in the aforementioned BLV viral trials. This is not a coincidence. L-forms (CWD forms) predominate and are crucial to the survival of mycobacteria in vivo and they have been documented by fluorescence microscopy in all intracellular macrophage-grown M. tuberculosis observed. From its origin, the very concept of the “BLV leukemic virus" has been on shaky, unstable ground. In 1969, veterinarians Janice and Lyle Miller from the University of Wisconsin-Madison spotted C-shaped “virus-like” particles in cattle lymposarcoma insisting that these were similar to other C-type viruses “regarded as the cause of leukemia in other species.” But by 1978, scientists at Downstate reported atypical mycobacterial forms, including its preferred filterable virussized “L” or cell-wall-deficient (CWD) forms in not only leukemia but all other malignancies ― all having, astheir common denominatorthe continuous presence of mycobacterial C-shaped forms. Tracing back to techniques similar to Miller and Millers original BLV study we found in the very lyophilized antigens present in commercial kits for the diagnosis of BLV (AgBLV), these very same CWD (cell-wall-deficient) mycobacteria and mycobacterial DNA in all BLV samples ― which when introduced into guinea pigs stimulated the same antibody as occurred when mycobacteria-infected internal organ homogenates themselves were injected into other guinea pigs. It is therefore assumed that the Bovine Leukemic Virus (BLV) is being mistaken for viral-like forms of cell-wall-deficient(CWD) atypicaltubercularmycobacteria. Since latent tubercular infection, as well as the administration of BCG and tuberculin also results in persistent CWD forms, their possible role in carcinogenesis is also considered.
https://bluecruiseturkey.co
https://bestbluecruises.com
https://marmarisboatcharter.com
https://bodrumboatcharter.com
https://fethiyeboatcharter.com
https://gocekboatcharter.com
https://ssplusyachting.com
Keywords
Cancer; Mycobacterium tuberculosis; Bovine leukemic virus; Mycobacteriophages
Introduction
BLV or the Bovine Leukemic Virus is a retrovirus. Peyton Rous was credited with the discovery and isolation of the first retrovirus. By 1911, Rous wanted to know why if one chicken got cancer, others followed [1]. Rous, who reproduced the malignant tumor at will in Plymouth Rock fowls, favoured a bacterial cause over a filterable virus. By 1933, Shope reported a viral tumor in cottontail rabbits, and Bittner reported on a milk-borne mouse breast cancer attributed to still other viruses [2]. With the advent of the electron microscope by the 1950s, particles later questionably ascribed to retroviruses were readily and routinely being detected. Indeed, as Dutch science historian Ton van Helvoort pointed out, by the 1950s the word "virus" had become so moldable a concept that one could speak of virus workers without the existence of any consensus whatsoever of what viruses were [3].
Post World War II investigators demonstrated that the retroviruses in the Rous, Bittner, and Shope tumours were actually filterable forms of mycobacteria [4]. Tuberculosislike, these “viruses” could be variably stained with acid-fast dyes and readily passed through a filter, but were found to be mycobacterial forms of the Actinomycetales having many of the characteristics of mycobacteria such as tuberculosis. Virginia Livingston of Rutgers, validated earlier work done proving that Rous’s virus was a bacteria, a finding soon validated by others [5-8].
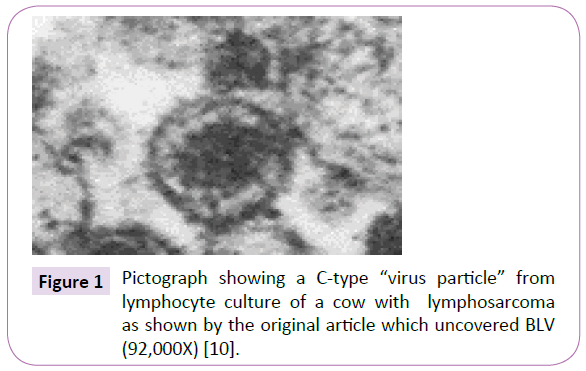
At the height of this battle over whether viruses or atypical tubercular mycobacteria caused cancer, nothing stood out more than the struggle over the etiology of the “C-shaped particle”. Miller and Miller, two of the veterinarians who “discovered” the BLV retrovirus in the form of C-shaped “virus-like particles” in cattle lymphosarcoma, insisted that their C-shaped particles were similar to other C-type viruses “regarded as the cause of leukemia in other species” [9,10] (Figure 1).
Figure 1 Pictograph showing a C-type “virus particle” from lymphocyte culture of a cow with lymphosarcoma as shown by the original article which uncovered BLV (92,000X) [10].
Two years later, Sorenson et al., saw leukemia as more accurately portrayed as not being transmitted by a virus, but an “infectious agent” [11].
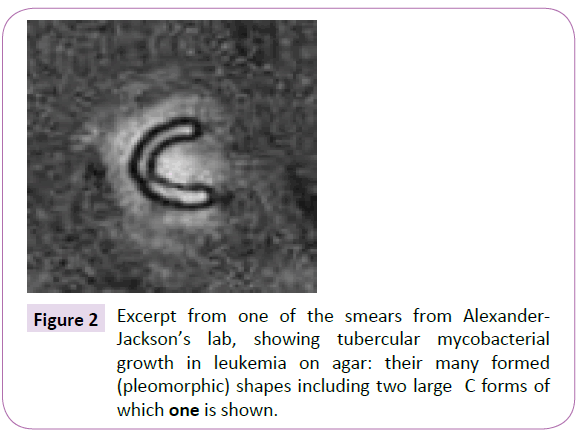
Although Robert Huebner, head of the NIAID at the time bought fully into the Miller’s C-shaped “virus” as the most likely cause of cancer ÃÆâÃâââ¬Ãâââ¬Â¢ to Dr. Eleanor Alexander-Jackson of Cornell, C-shaped ‘virus-like particles’ had nothing to do with viruses like BLV at all, ÃÆâÃâââ¬Ãâââ¬Â¢ rather they represented round tubercular mycobacterial L-forms that split into a characteristic “C”, stainable by special mycobacterial stains, one of which she herself had created ÃÆâÃâââ¬Ãâââ¬Â¢ her “triple stain” [12] (Figure 2).
Bacterial L-forms, the connecting link between viruses and bacteria, were first described by Klieneberger at England’s Lister Institute, for which she named them “L-forms” [13]. Such bacteria were ‘cell-wall deficient’ (CWD) because they either had a disruption in, or a lack of, a rigid bacterial cell wall. This lack of rigidity allowed bacteria or mycobacteria the plasticity to assume many forms (pleomorphic), some of them virus-like, but all of them different from their classical parent. Such forms were also poorly demonstrated by ordinary staining, while many of them, just like viruses, easily passed through the finest of filters. Of all the bacteria, it is tubercular L-forms (cell-wall-deficient forms) that predominate and are crucial to the survival of tuberculosis and the mycobacteria [14]. It is mostly in its cell-wall-deficient forms that TB can escape destruction by the body’s immune system. And at the same time, CWD forms of the tuberculosis-like mycobacteria react similarly in ELISA blood tests to retroviruses such as HIV, which they can simulate in every way [15].
By the twentieth century, polymorphic, partially acid-fast (AF), cell-wall-deficient (CWD), filter passing forms of tuberculosis were being isolated from tumor homogenates and the blood of leukemic patients [16-24]. But actually, the striking similarity between cancer and tuberculosis-like microorganisms was noticed in 1877 by Sir John Simon ÃÆâÃâââ¬Ãâââ¬Â¢ long before the tubercle bacillus was even discovered. Research in the last two decades does nothing but confirm this. Repeatedly, recent studies have shown atypical tubercular cell-wall-deficient forms (also known as “L-forms”), and tubercular DNA integrated into the genome of malignant tissue [25-34]. However, since tuberculosis and the development of a malignant tumor can proceed at the same time, more objective data can be obtained studying tumor cells cultivated in vitro [35]. One convenient way to do this is to study fetal lamb kidney cells infected with the Bovine Leukemic Virus(FLK-BLV), whose antigens are available in irradiated filtered commercially available kits for the purpose of detecting the Bovine Leukemic Virus (BLV).
Despite its mixed history, leukemia in cattle is presently thought to be caused by the purportedly oncogenic bovine leukemic virus (BLV) of the Retroviridae family which is said to cause a neoplastic proliferation of hematopoietic and lymphoid tissues that to a certain extent remains a problem for cattle breeding. Diagnostics for bovine leukemia are conducted by the detection of the antibody of BLV, in particular by the agar gel immunodiffusion (AGID) test, using concentrated FLK-BLV culture as the antigen (AgBLV). Yet the existence of a relationship between the retroviruses and tuberculosis has long been known. Alexander- Jackson mentioned a tubercular-like microbe artificially cultured from the blood and tumors of all Rouse retrovirus (specifically called the “Rouse agent” by Rouse himself) infected chickens [34]. Similarly Jackson isolated cultures similar to cell-walldeficient (CWD) tuberculosis from human leukemia [20].
Just as it is known that injection with tuberculin in cattle increases the level of antibodies to antigens of BLV and that these same BLV antigens in turn have a cross-reactivity with mycobacterial antigens ÃÆâÃâââ¬Ãâââ¬Â¢ it is also known that the p55 and p24 in the HIV-1 retrovirus can react with the blood of people sick with leprosy and tuberculosis [36,37]. For all of these reasons, the purpose of this study was to investigate a series of commercially available antigen diagnostics presently available to detect bovine leukemia (AgBLV) for the possible presence of mycobacteria in them.
Materials and Methods
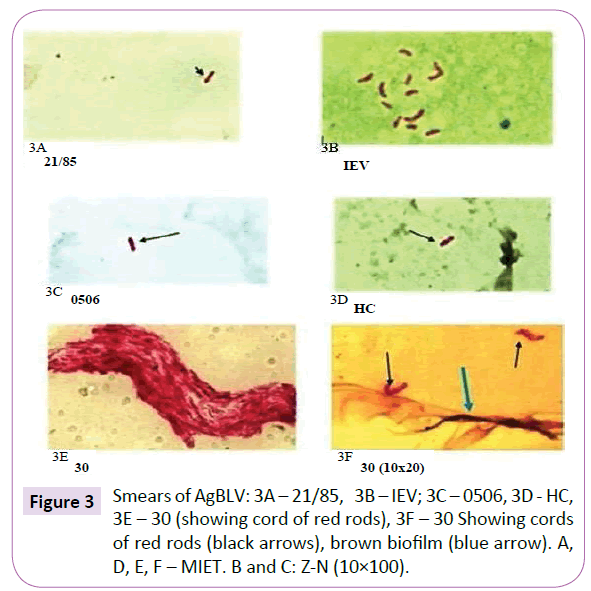
Twelve lots of AgBLV for AGID made in the Russian Federation of Belarus from 1985 to 2013 were used (Table 1). Commercially available lyophilized AgBLV was dissolved in growth stimulant MycCell DW, of which 0.15 ml was distributed on standard glass slides [38,39]. Dried smears were fixed at 65oC with 3% H2O2 and 95% methanol to inactivate endogenous peroxidase. This was followed by Ziehl-Neelsen and Kinyoun staining as well as a modified immunoenzyme technique (MIET) that colors acidfast (AF) tubercular forms in red; non-acid-fast (NAF) forms in shades of brown, and non-tubercular microflora and tissues in blue [39,40]. Results were recorded using an Olympus 56BX microscope.
| Lot numbers of AgBLV | Microscopy of AbBLV smears(10×100) | Growth of AgBLV on MycCel DW | PCR of DNA isolates with primers 16S rRNA |
|---|---|---|---|
| 21/85 | 4 AF rods, brown NAF biofilm | + | Not investigated |
| IEV | 20 AF rods, brown NAF biofilm | + | + |
| BN | 3 AF rods, brown NAF biofilm, 5 brown rods | + | + |
| 0805 | 3 AF rods, brown granular forms | + | Not investigated |
| 0506 | 4 AF rods | + | + |
| 0806 | 2 AF rods, brown NAF biofilm, red granular forms | + | + |
| HC | 3 AF rods, brown NAF biofilm | + | + |
| 2 R | Clusters of red granular forms surrounding brown halos | + | + |
| 3 | Brown NAF biofilm and granular forms | + | + |
| 27 | 3 AF rods, brown NAF biofilm | + | + |
| 30 | Clusters of AF rods, brown biofilms | + | + |
| 38 | Cluster AF rods brown rods and “network” | + | + |
Table 1 Examined Lots of AgBLV.
AgBLV was dissolved in growth stimulant and then incubated for 48 hours at 37°C. After that 0.3-0.5 ml of each mixture was placed in a mycobacterial nutritive medium (MycCel DW), and incubated for 10-15 days at 37°C. Resulting colonies were then planted on the same nutritive medium [39,40].
The bacterial mass of the two isolates from AgBLV lots BN and HC (randomly selected), were incubated for 15 min in 6% oxalic acid (1 mg/ml), centrifuged, and then suspended in the growth stimulant for 24h. In a second phase, these same two bacterial isolates were incubated for 24 h in the same growth stimulant with 0.15% chlorhexidine before being sown on the same growth medium.
To determine the pathogenicity of isolates obtained from AgBLV, we used the isolates from lots BN and HC grown on 6% oxalic acid admixed with 0.5 ml of mineral oil were hypodermically administered to guinea pigs as a first passage. After 75 days an autopsy was performed and 1.5 ml homogenates of their internal organs were injected into new guinea pigs for a second passage. This was repeated for a total of four passages. Homogenates of internal organs decontaminated by 6% oxalic acid were then incubated in growth stimulant and sown on a nutrient medium (MycCel DW).
With regard to antisera, purified antibody and blood serums, we used:
1.The conjugated antibodies to M. bovis Valle with peroxidase Sigma Type IV, previous to which the rabbit antiserum was absorbed by non-tubercular microflora, and antibody was purified on Affi-gel 10 (Bio Rad Laboratories) with antigens of the M. bovis sonicate.
2. Rabbit antiserum to CWD (Cell-Wall-Deficient) M. bovis 8.
3. Sheep antiserum to CWD M. tuberculosis H37Rv.
4. Six sera from leukemic cows (AGID positive).
5. Three sera from healthy guinea pigs.
Agar Gel Immunodiffusion (AGID) tests were done according the technique in the OIE Manual (2009) whereas SDS-PAGE electrophoresis was done in 15% of PAGE [41]. Bacterial mass isolates were destroyed by warming them for 5 minutes at 100°C in a lysis buffer.
Polymerase Chain Reaction (PCR): Isolates from AgBLV (0.2- 0.5 mg/ml) were warmed-up for 5 min (95°C) in the lysis buffer. The DNA was isolated on columns with a sorbent (IBOH NANB) and amplification was carried out on a C1000 TouchTM Thermal Cycler (BioRad) with 16S rRNA mycobacterial primers (Praymtech) according to standard protocols. An electrophoresis of amplificates was then carried out in 2% agarose gel and results obtained from a Molecular Imager®GelDocTM XR+system (BioRad).
Results
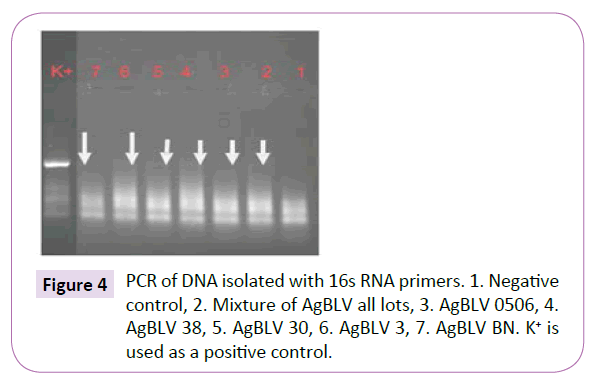
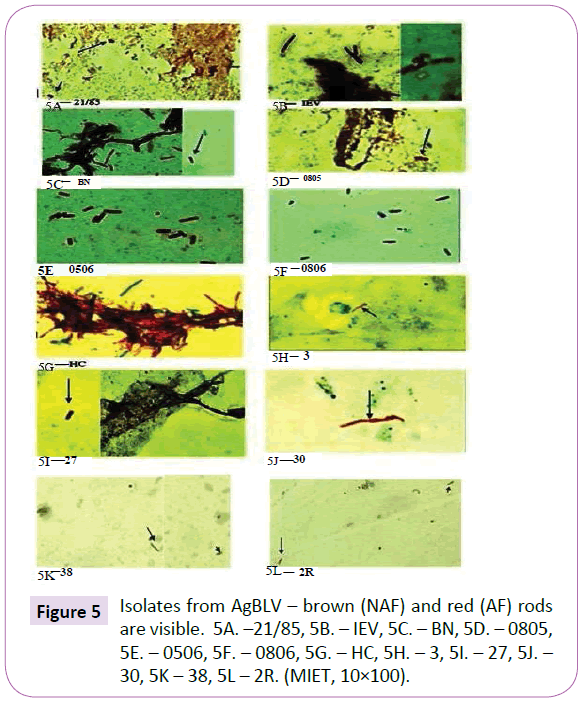
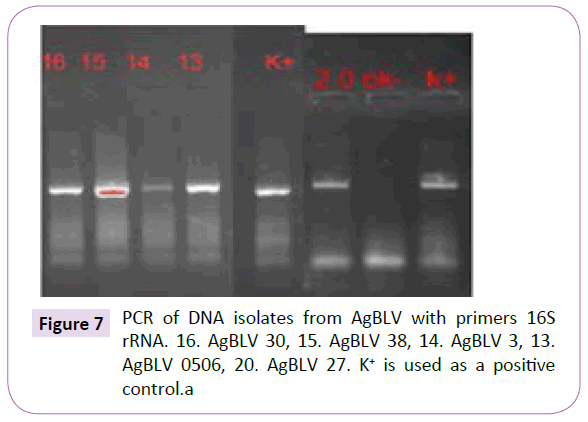
Out of a total of 12 smears performed on commercial samples of AgBLV, 10 of them (83%) stained acid-fast (AF) positive as classic red tubercular rods. In addition, and more widely prevalent were non-acid-fast (NAF) forms (rods, cocci, filaments), which reacted with antibodies to M. bovis and stained brown with MIET (Table 1) (Figures 3-15). In addition to this PCR mycobacterial primers for 16S rRNA confirmed the presence of mycobacterial DNA in standard BLV antigens (AgBLV) used to detect Bovine Leukemic Virus (Figures 4, 6 and 7). All samples of AgBLV that were first incubated in a mycobacterial growth stimulant (48 hours at 37° C) and then seeded on MycCel DW medium for from 4 to 14 days yielded NAF (non-acid fast) mycobacterial rods and coccoid forms (Figure 5). These, along with most of the 10 lot numbers of AgBLV that had classical AF tubercular rods were confirmed by PCR with mycobacterial primers to 16S rRNA (Figures 6 and 7).
Figure 6 PCR of DNA with primers 16S rRNA, 10, 20, 30, using primary growth from first and second passage isolates of AgBLV IEV. 40 – Shows isolate from bovine tuberculin, 50 - Negative control, a marker of m. m.; K+ - positive control; 8. CWD M. bovis Vallee, 9. M. bovis Vallee, 10ÃÆêÃâà ¾Ãâââ¬Â¹– Native AgBLV HC, 10 m. Native AgBVL IEV.
In addition, using AGID, sonicates of isolates from AgBLV were noted to have identical antigens to those of M. bovis (Figure 8).
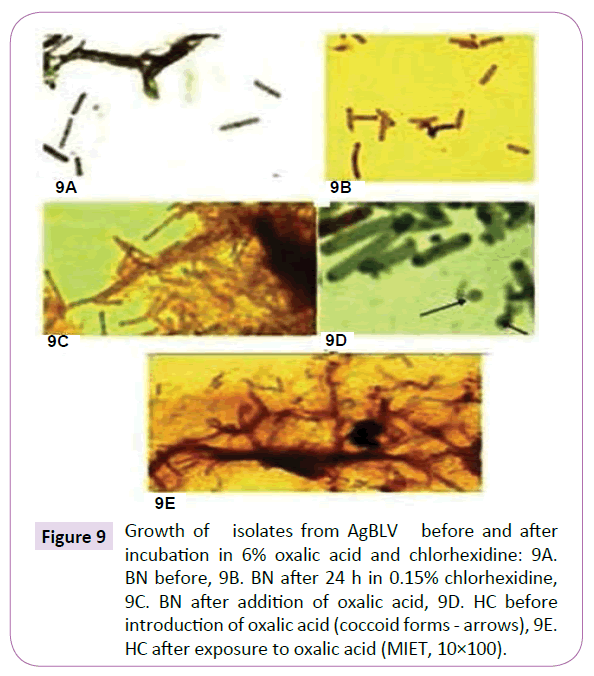
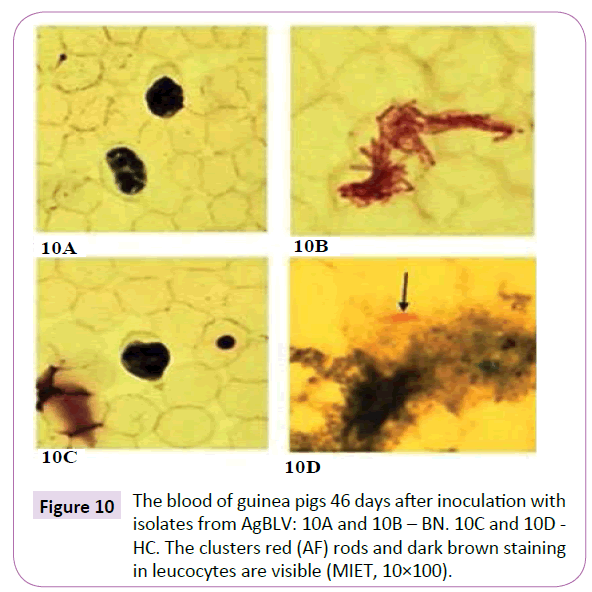
Practically indestructible, mycobacterial isolates from AgBLV, BN and HC both kept their viability, albeit with different morphology from our original cultures, after being subjected to 6% oxalic acid and 0.15% chlorhexidine solution for 24 hours before placement in MycCel DW growth medium for 3-4 days (Figure 9). Furthermore, when isolates from BN and HC were injected into guinea pigs after being subjected to 6% oxalic acid, cell-wall deficient forms not only remained alive for the full 75 days that specimens were observed, but within 46 days displayed classic red (AF) tubercular rods in their respective blood leukocytes (Figure 10).
Figure 9 Growth of isolates from AgBLV before and after incubation in 6% oxalic acid and chlorhexidine: 9A. BN before, 9B. BN after 24 h in 0.15% chlorhexidine, 9C. BN after addition of oxalic acid, 9D. HC before introduction of oxalic acid (coccoid forms - arrows), 9E. HC after exposure to oxalic acid (MIET, 10×100).
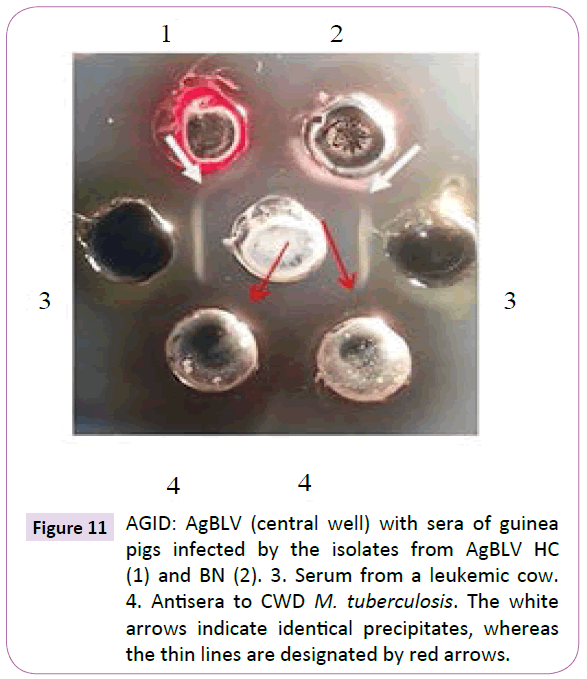
Guinea pig blood from the first passage reacted in AGID with AgBLV which in turn formed precipitates merging with those precipitates formed by the serum of a sick leukemic cow. These bovine leukemic precipitates then merged with precipitates formed by adding them to the antiserum of cell-wall-deficient (CWD) M. tuberculosis (Figure 11). During first passage guinea pig autopsies, the only lesions visible were at the injection site and inguinal lymph nodes (lymphadenopathy).
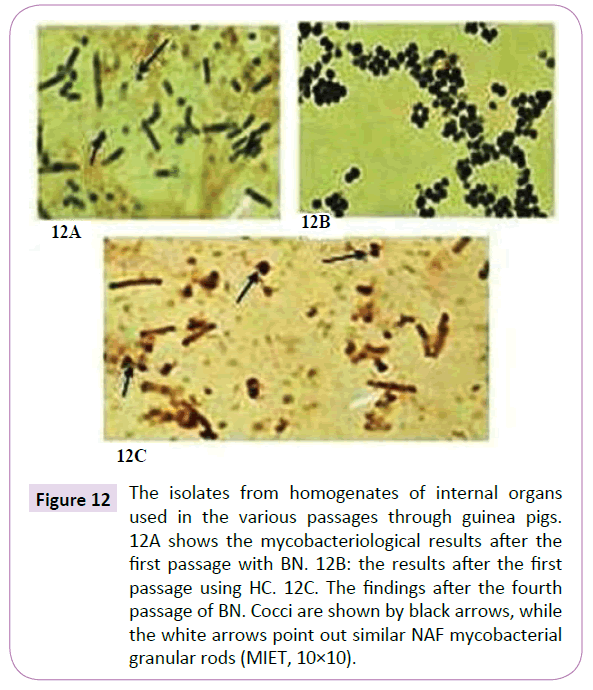
After that initial passage from homogenates of the organs of guinea pigs in first passage of cultured samples ÃÆâÃâââ¬Ãâââ¬Â¢ those from BN produced granular rods and cocci whereas isolates from HC contained cocci (Figure 9D) Isolates were specifically stained by the MIET or differential immunoperoxidase method. Their identity as mycobacteria was confirmed by PCR (Figure 13). And both cultures were, from the first passage sensitive to firstline TB drugs such as Isoniazid, rifampicin, pyrazinamide, and ethambutol.
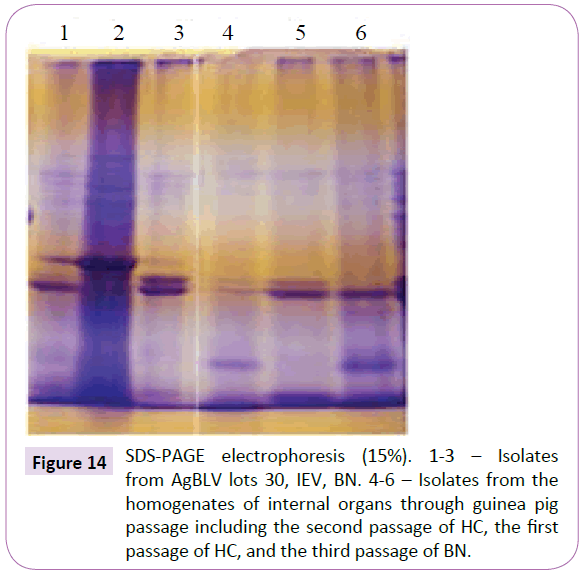
As a second passage animal organ homogenates from the first passage were injected into uninfected guinea pigs. Using intervals of from 75 to 80 days, third and fourth passages of these organ biomaterials were carried out. Although in all cases rods and cocci could be identified through appropriate mycobacterial stain, there existed a noticeable difference in morphology from those cultures isolated from the initial passage (Figures 12A and 12C). Nevertheless that these variant forms were mycobacterial was confirmed through PCR (Figure 13). And they had polypeptide spectrums almost identical and not much differing from those spectrums obtained from native isolates of AgBLV (Figure 14).
Figure 12 The isolates from homogenates of internal organs used in the various passages through guinea pigs. 12A shows the mycobacteriological results after the first passage with BN. 12B: the results after the first passage using HC. 12C. The findings after the fourth passage of BN. Cocci are shown by black arrows, while the white arrows point out similar NAF mycobacterial granular rods (MIET, 10×10).
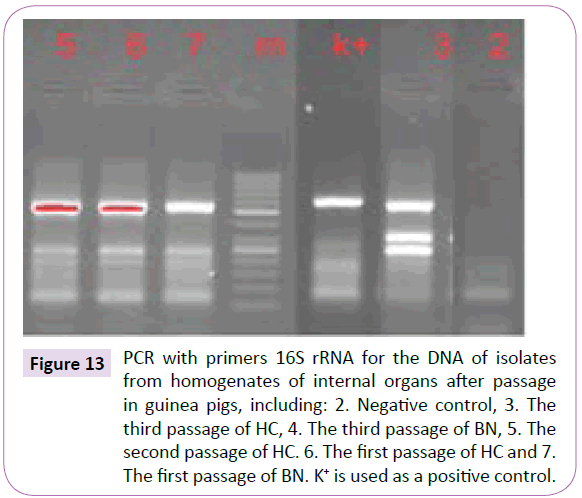
Figure 13 PCR with primers 16S rRNA for the DNA of isolates from homogenates of internal organs after passage in guinea pigs, including: 2. Negative control, 3. The third passage of HC, 4. The third passage of BN, 5. The second passage of HC. 6. The first passage of HC and 7. The first passage of BN. K+ is used as a positive control.
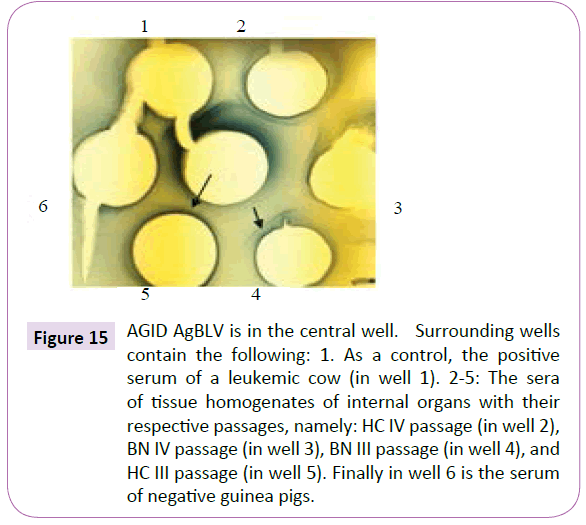
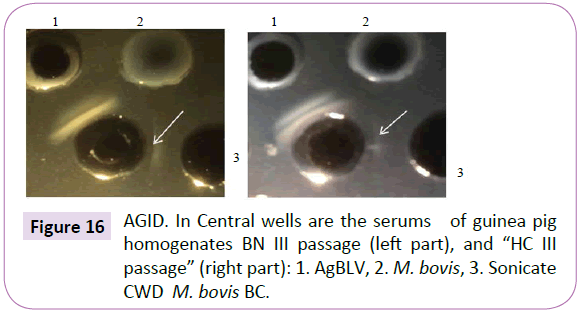
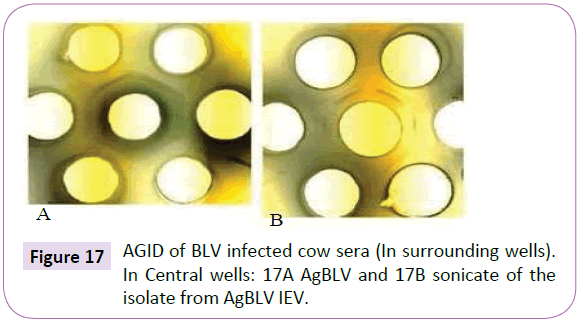
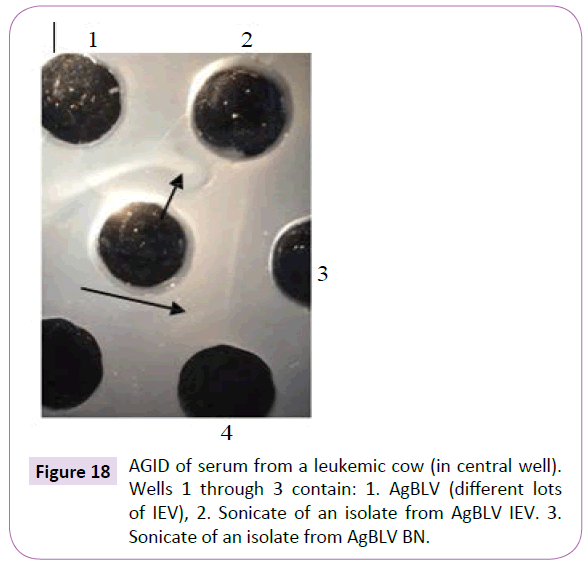
Notice that sera from the second, third and fourth guinea pig passages, which reacted in AGID with AgBLV, not only gave lines identical or partially identical with the precipitate of serum from a sick leukemic cow ÃÆâÃâââ¬Ãâââ¬Â¢ but they also reacted with the sonicate of CWD M. bovis BCG (Figures 15 and 16). In addition, sonicates isolated from AgBLV reacted with the sera of leukemic cows as well as commercially standardized AgBLVS with the formation of smoothly merging precipitates or partially merging precipitates (Figures 17 and 18). Of interest is that serum from the fourth passage not only reacted with AgBLV, giving lines similar to isolated sonicates from AgBLV, but findings identical to those shown for tuberculin (Figure 21).
Figure 15 AGID AgBLV is in the central well. Surrounding wells contain the following: 1. As a control, the positive serum of a leukemic cow (in well 1). 2-5: The sera of tissue homogenates of internal organs with their respective passages, namely: HC IV passage (in well 2), BN IV passage (in well 3), BN III passage (in well 4), and HC III passage (in well 5). Finally in well 6 is the serum of negative guinea pigs.
Discussion
We found, in all cases, acid-fast [AF] and non-acid-fast [NAF] mycobacterial forms in addition to mycobacterial DNA in commercially prepared, widely used kits containing AgBLV (BLV antigen) to diagnose professedly cancer-causing BLV. This was despite the gamma irradiation and the filtering of culture media common to the production of such standardized BLV antigen kits. In addition our cultures and diagnostics of the lyophilized biomaterials in these kits ÃÆâÃâââ¬Ãâââ¬Â¢ based on morphology, acid-fastness, common antigens and PCRs with mycobacterial 16S rRNA left little doubt regarding the findings of classic and cell-wall-deficient tubercular mycobacteria which cultures out on fetal lamb kidney cells (FLK). Our results indicate that BLV is merely a viral-like mycobacteria capable of cultivation on FLK, resulting in either cell-wall-deficient (CWD) or acid-fast (AF) mycobacterial forms.
Duesberg, who did much of the pioneer work on retroviral ultrastructure, never believed in the cancer-causing potential for retroviruses like the Bovine Leukemic Virus (BLV). Rather he mentions that retroviruses are rather widespread in healthy animals and humans where they typically cause latent infections and antiviral immunity and that “The leukemia risk of such [retroviral] infections is less than 0.1% and thus about as low as that of virus-free controls [42].” And even as far back as 1957, Dmochowski, a virologist and electron microscopist at The University of Texas M.D. Anderson Hospital and Tumor Institute warned this about attributing “virus-like” particles such as found in the Miller’s original ‘discovery’ of Bovine Leukemic Virus (BLV) as the direct cause of cancer. Dmochowski and Grey said this:
“Unfortunately, we are far from understanding the significance and meaning of the structure of viral particles……no matter how exciting electron microscope pictures of subcellular particles may look, their significance can be properly assessed only if combined with suitable biological, biochemical, and other studies. This point is particularly pertinent to studies on subcellular particles of virus-like appearance that may be found in neoplastic cells ”[43].
In her own review of this subject thirteen years later Seibert mentions the cancer findings of Demochowski and Grey as well as those of Dameshek in an altogether different light [43,44]. These viral studies, pointed out Seibert, found inclusion bodies measuring from about 0.25-1.7 μ in the tissues and blood cells of patients or animals with leukemia or other malignancies [45]. These inclusion bodies in turn had “virus-like” inclusions of about 0.03 μ. Furthermore, it was not only striking to Seibert how closely such “virus-like” measurements approximated the sizes of variably acid-fast bacterial forms that she had isolated in all cancers [0.26-1.7 μ], but also the tiny inclusions that she likewise saw within these mycobacterial variably acid-forms ÃÆâÃâââ¬Ãâââ¬Â¢ measuring about 0.01-0.05 μ were similar in size, to certain phage ÃÆâÃâââ¬Ãâââ¬Â¢ specifically the same mycobacteriophage that Mankiewicz not only found in isolates from malignant tissues, but that were responsible for malignant change in those mammalian tissues [46].
Seibert would go on to say:
"One of the most interesting properties of these bacteria [in all malignancies] is their great pleomorphism [ability to change form]. For example, they readily change their shape from round cocci, to elongated rods, and even to thread-like filaments depending upon what medium they grow on and how long they grow. This may be one of the reasons why they have been overlooked (as cancer’s cause) or considered to be heterogeneous contaminants ... And even more interesting than this is the fact that these bacteria have a filterable form in their life cycle; that is, that they can become so small that they pass through bacterial filters which hold back bacteria. This is what viruses do, and is one of the main criteria of a virus, separating them from bacteria. But the viruses also will not live on artificial media like these bacteria do. They need body tissue to grow on. Our filterable form, however, can be recovered again on ordinary artificial bacterial media and will grow on these. This should interest the virus workers very much and should cause them to ask themselves how many of the viruses may not be filterable forms of our bacteria [47]."
Seibert’s cancer attentions were therefore not only riveted on pleomorphic, variable acid-fast, cell-wall-deficient (CWD) tubercular-like forms within her cancer tissue studies, but minute inclusions representative of mycobacteriophage. Mycobacteriophages are the virus within all virulent infections of the Actinomycetales, whether tubercular, mycobacterial or related variable acid-fast species. And it was at this point that Florence Seibert speculated as to just how the nuclear material of mammalian cells and tissues could become vulnerable to malignant transformation during their prolonged and apparently symbiotic residence in the body. Specifically, on the subject of bacteriophage inspired carcinogenesis, Seibert modeled what she felt happened along the lines of prophage activation.
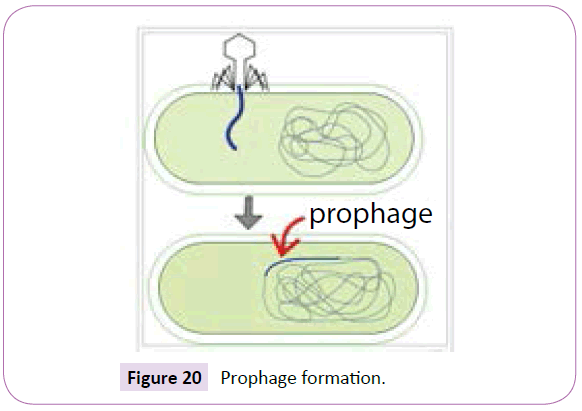
A prophage is the genome of a bacteriophage or mycobacteriophage (often simply called “phage”) which can incorporate into a bacteria’s DNA when phage lands on and attacks a bacteria, injecting its nuclear material into the bacteria when it pierces a bacteria’s cell wall (Figure 20).
Phage activation occurs when an insult or stress, such as with Ultra-Violet (UV) light (which is also a human carcinogen) causes its bacterial host damage. When this happens, the prophage of phage genetic material, already lodged in bacterial chromosomes is then excised from them and can go into a reproductive cycle in which the viral phage commandeers the bacterial cell’s reproductive machinery. Seibert and Mankiewicz, by merely directing attention to the cytopathogenic effects of mycobacteriophage on mammalian and therefore human tissue, were merely pointing out what should have been an obvious mechanism behind cancer. Specifically, phage seemed to be directly affecting mammalian cell reproduction in a similar way to its actions on bacteria, but in this case eventually causing unmitigated human cell division [45,46].
Much of the recent work regarding TB and its related microorganisms as causative of cancer has dealt with the “L” or “cell-wall-deficient” forms of typical or atypical Mycobacterium tuberculosis. Cell-wall-deficient variants can produce and eject as much as 12 to 23 times as many phage units into mammalian tissue as comes from their parent microbes [48,49].
Recently Falagas reviewed 14 case reports that evaluated the history of TB as a risk factor for the development of a malignant tumor [50]. But the median time between the initial appearance of TB infection and the metachronous [appearing at different intervals] detection of malignancy averaged to be greater than 20 years, except in a single case that reported cancer development after only 11 months of infection. Falagas et al.’s data strongly suggests that TB and various types of malignancies are able to mimic each other and that even if biopsy specimens are malignant, they should also be sent for stain and culture for M. tuberculosis ÃÆâÃâââ¬Ãâââ¬Â¢ a practice, which, as in the various BLV trials, is practically never followed in laboratory or clinical studies. Falagas reported on approximately 91 cases in which TB seemed to masquerade as malignant tumors. Most of these were based on clinical or radiologic evidence of a malignancy where histopathology revealed only mycobacteria.
What is the nature and origin of these L-forms or cell-walldeficient forms of the tubercular mycobacteria that have captured the majority of attention in recent cancer studies?
Nelson and Pickett long maintained that such cell-wall-deficient (CWD) states were largely the result of bacteriophage or in the case of the mycobacteria, mycobacteriophage attack with the other purported causes simply enhancing phage activity to throw the cell into a cell-wall-deficient state [51]. According to Nelson, why bacteria or mycobacteria became 'cell-wall-deficient' and therefore assumed many forms was simply the battering that their bacterial cell walls took from phage attack. Although Nelson and Pickett acknowledged the many conditions that were said to contribute to cell-wall deficiency, they saw all of these other agents as simply activating such phage intrusion. For example, they saw Kruger's work with penicillin, a known cause of cellwall- deficient forms, as just another substance that enhanced phage activity [52]. Moreover Nelson and Pickett showed that such phage attack could keep a microorganism locked in a cellwall- deficient state indefinitely, a finding which dovetailed with Takahashi's Japanese study of the different cell-wall-deficient forms of tuberculosis [51,53].
The role of mycobacteriophages, the viruses which live in all virulent tubercular and CWD mycobacteria has always been underestimated in the genesis of cancer. Livingston noted that there was no viral agent that was equivalent to the phage which has been proven to be causative in cancer [4]. This was only after Mankiewicz showed that mycobacteriophages themselves could instigate pre-malignant human tissue change – the same mycobacteriophages that could be activated by a host of other environmental carcinogens, which essentially activated phage leading to their multiplication, extrusion onto mammalian tissue and the cytopathologic changes which this caused [46].
Further evidence for the role of CWD (cell-wall-deficient) mycobacteria and their mycobacteriophages in cancer lies in the ability of one mycobacteria, such as BCG (dilute Mycobacterium bovis) to combat cancer in the same manner one mycobacteria (M. smegmatis), transfected with the appropriate mycobacteriophage can kill other mycobacteria, such as virulent strains of M. avium and M. tuberculosis [54]. Currently, bacillus Calmette-Guérin (BCG), a live attenuated strain of Mycobacterium bovis, is the only agent approved by the US Food and Drug Administration as the primary therapy of carcinoma in situ (CIS) of the bladder. Studies have consistently shown that BCG treatment can eradicate this cancer in 70% of patients with CIS of the bladder when used correctly, though to prevent cancer recurrence, long-term maintenance therapy following the induction phase is necessary.
Although the mechanism of action of BCG therapy is incompletely understood, once in the bladder, the live organisms enter macrophages, where they induce the same type of histologic and immunologic reaction as found in patients with tuberculosis. BCG vaccine ostensibly enters bladder cancer cells, where a direct cellto- cell cytotoxic response seems to target these cancer cells for destruction. The precise mechanism as seen by a 1993 University of Illinois study was that initially the cancer cells seemed to eat (or phagocytize) and thus kill the Mycobacteria bovis in BCG. But then, suddenly, the cancer cells too died. Although investigators in the study admitted the relationship wasn’t clear, again, a strong ‘tumoricidal agent’ inside the Mycobacteria bovis or cow tuberculosis was pointed to, but obviously such death to the malignant cells could have been caused by BCG’s phage weaponry [55].
Thus, what these investigators were really probably looking at was a common phenomenon in nature known as ‘lysogeny’. Lysogeny is what happens when one colony of a similar bacteria kills another by hurling viral phage arsenal towards it and this, and the fact that phages are usually species specific, is what Virginia Livingston was referring to when she said that it seemed to her that the rationale behind why BCG was effective not only against tuberculosis but cancer was that both originated from closely related germs, and that ultimately the phages released by the Mycobacteria in BCG were therefore able, to one extent or another, effectively kill her modified mycobacterial cancer germ that she felt was behind cancer.
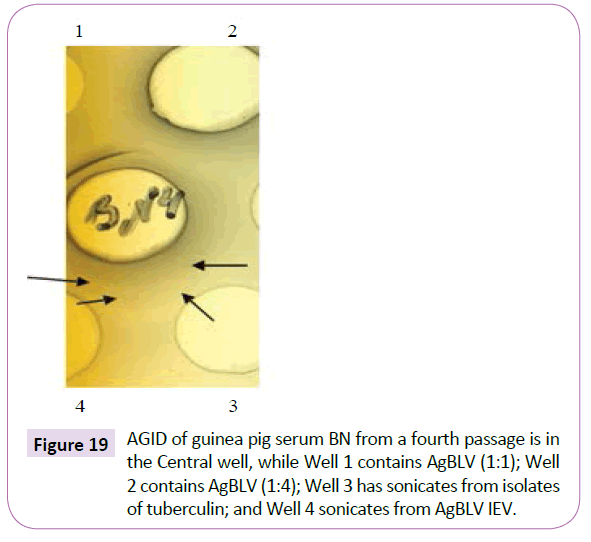
In the present study, the growth medium MycCel DW, allowed the isolation from AgBLV and from subsequent guinea pigs passages CWD tubercular cultures similar to isolates obtained from tuberculin (Figure 21). And Sera from such guinea pig passage reacted with both sonicates from AgBLV and tuberculin isolates with formation of identical lines (Figure 19).
Since latent tubercular infection, as well as the administration of BCG and tuberculin also result in persistent tubercular CWD forms which can discharge phage systemically ÃÆâÃâââ¬Ãâââ¬Â¢ their possible role in carcinogenesis, although not proven, cannot be altogether ignored [38,56]. Seibert speculated that it was the nuclear material of mammalian cells and tissues infected with such pleomorphic CWD bacteria that could, over time, become vulnerable to malignant transformation either through contact with the chromatin material of filterable CWD particles or their virulent mycobacteriophages. Whether latent or active or from the mere introduction of tuberculin or the BCG ÃÆâÃâââ¬Ãâââ¬Â¢ mycobacteria, once housed in the human macrophage assume cell-walldeficient forms [57].
Such a potential for even latent forms of CWD to eventually instigate carcinogenesis of course depends upon the future virulence of the dormant microbes introduced into the body. Yet phage, which can actually figure into the eventual pathogenicity of TB, is once again instrumental here. Having the ability to alter many bacterial or mycobacterial properties, phages can alter all stages of the infectious process, including bacterial adhesion, colonization, increase of virulence, invasion, spread through human tissues; resistance to immune defenses; sensitivity to antibiotics; and transmissibility among humans [58].
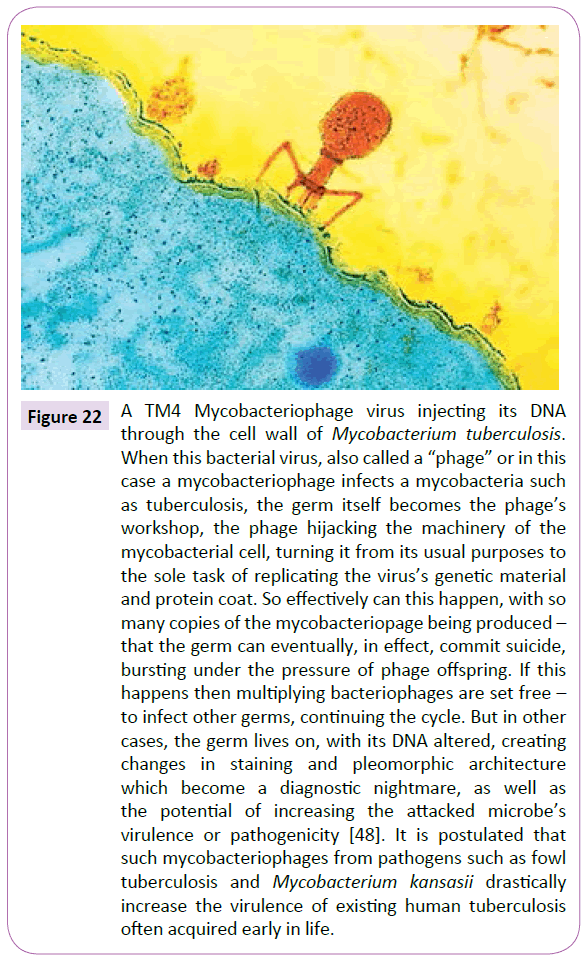
The ability of CWD tubercular forms to have the ability to spring back to lethal classic forms, even in the face of the most extreme environmental conditions in a real sense makes them ‘immortal.’ Falagas has indicated that the average latency between mycobacterial infection and the induction of cancer is approximately 20 years [49]. We also know that latent or dormant TB maintains the right to become active virulent TB. In the meantime it is not outside the realm of reason to say that it is not impossible that any tubercular content introduced into the system cannot through phage intercession be integrated into the cells and tissues of the body resulting in certain instances in the uncontrollable reproduction of the cells and tissues of our body (Figure 22).
Figure 22 A TM4 Mycobacteriophage virus injecting its DNA through the cell wall of Mycobacterium tuberculosis. When this bacterial virus, also called a “phage” or in this case a mycobacteriophage infects a mycobacteria such as tuberculosis, the germ itself becomes the phage’s workshop, the phage hijacking the machinery of the mycobacterial cell, turning it from its usual purposes to the sole task of replicating the virus’s genetic material and protein coat. So effectively can this happen, with so many copies of the mycobacteriopage being produced – that the germ can eventually, in effect, commit suicide, bursting under the pressure of phage offspring. If this happens then multiplying bacteriophages are set free – to infect other germs, continuing the cycle. But in other cases, the germ lives on, with its DNA altered, creating changes in staining and pleomorphic architecture which become a diagnostic nightmare, as well as the potential of increasing the attacked microbe’s virulence or pathogenicity [48]. It is postulated that such mycobacteriophages from pathogens such as fowl tuberculosis and Mycobacterium kansasii drastically increase the virulence of existing human tuberculosis often acquired early in life.
This study contradicts prevalent current ideas on the etiology of tumors and malignancy, particularly with regard to bovine leukemia, but not to the exclusion of other cancers. We have suggested a core cause for many cancers ÃÆâÃâââ¬Ãâââ¬Â¢ as the mycobacteria, related Actinomycetales, and the phages within them, all offers a likely prophage mechanism involved in carcinogenesis. Nor, should the fact that secondary ‘viruses’ and bacteria are being picked-up in certain cancer studies come as a surprise to readers as Koch himself taught that more often than not tuberculosis is accompanied by secondary pyogenic bacteria.
Even if BLV is once and for all recognized as a product of the variability of TB, in no way does its persistence in the body alone always initiate oncogenesis. Factors such as the condition of the immune system, the quantity and the pathogenicity of strains involved and many other factors are at work. Nevertheless, the persistence of potentially lethal and potentially oncogenic CWD forms of TB and related species of the Actinomycetales ÃÆâÃâââ¬Ãâââ¬Â¢ either through infection or vaccination necessitates its continued study and surveillance for cancer development on a global scale. For latent, asymptomatic, CWD infection does not preclude the incessant bombardment of mammalian tissue with potentially oncogenic phage.
References
- Rous PA (1911) Sarcoma of the fowl transmissible by an agent separable from the tumour cells. J Exp Med 13: 397-411.
- Bittner JJ (1936) Some possible effects of nursing on the mammary gland tumour incidence of mice. Science 84: 162.
- Van Helvoort T (1994) History of virus research in the 20th century: the problem of conceptual continuity. History of Science 32: 185-235.
- Livingston V (1972) Cancer: A New Breakthrough. Nash Publishing p: 269.
- Livingston V (1970) Specific type of organism cultured from malignancy: bacteriology and proposed classification. Ann NY Acad Sci 174: 636-654.
- Duran-Reynals F (1950) Neoplastic infection and cancer. Am J Med 8: 440-511.
- Glover T, Scott MA (1926) Study of the Rous chicken sarcoma no 1. Canada Lancet Pract 66: 49-62.
- Diller I (1970) Experiments with mammalian tumor isolates. Ann NY Acad Sci 174: 655-674.
- Miller JM, Miller LD, Olson C, Gillette KG (1969) Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst 43: 1297-1305
- Miller JM (1974) Animal model of human disease. Malignant lymphoma. Am J Pathol 75: 417-420.
- Sorensen DK, Dutta SK, Hammer RF, Larson VL, Perman V, et al. (1970) Bovine lymphocytic leukemia: studies of etiology, pathogenesis and mode of transmission. Progress Report no. 10 to the U.S. Atomic Energy Commission, 1969-1970 p: 38
- Alexander-Jackson EA (1954) specific type of microorganism isolated from animal and human cancer: bacteriology of the organism. Growth 18: 37-51.
- Klieneberger-Nobel E (1949) Origin, development and significance of L-forms in bacterial cultures. J Gen Microbiol 3: 434-442.
- Mattman LH (2000) Cell wall deficient forms: stealth pathogens. CRC Press.
- Kashala O, Marlink R, Ilunga M, Diese M, Gormus B, et al. ( 1994) Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J Infect Dis 169: 296-304.
- Glover T (1930) The bacteriology of cancer. Canada Lancet Pract 75: 92-111.
- Mazet G (1941) Etude Bacteriologique sur la Maladie d’ Hodgkin. Montpellier Med pp: 1-6.
- Livingston V, Allen R (1948) Presence of consistently recurring invasive myco- bacterial forms in tumor cells. Microscop Soc Bull 2: 5-18.
- Wuerthele-Caspe V (1949) Mycobacterial forms observed in tumors. J Am Med. Womens Assoc 4: 135-141.
- Alexander-Jackson E (1976) Progenitor Cryptocides, The Specific Pleomorphic Microorganism Isolated From Cancer. J Int Acad Metab 5: 31-39.
- Alexander-Jackson E (1978) Microscopic and Submicroscopic Phases of P. Cryptocides from Fresh Lymphocytic leukemia. J Int Acad Metab 1: 9-18.
- Diller I, Diller W (1965) Intracellular acid-fast organisms isolated from malignant tissues. Trans Am Micr Soc 84: 138-148.
- Diller I, Donnelly A, Fisher M (1967) Isolation of pleomorphic, acid-fast organisms from several strains of mice. Cancer Res 27: 1402-1408.
- Seibert F, Feldmann F, Davis R, Richmond I (1970) Morphological, biological, and immunological studies on isolates from tumors and leukemic bloods. Ann N Y Acad Sci 174: 690-728.
- Wang A, Xie J (1998) Infection of mycobacterium tuberculosis in lung cancer. Zhongguo Fei Ai Za Zhi 1: 92-94.
- Guliang H, Tefu L (1999) Mycobacterium tuberculosis L-forms. Microb Ecol Health Dis 10: 129-133.
- Xie J, Anchao W, Xiazhi Z (1999) Isolation of acid fast bacillus L- forms from carcinoma of Lung. Acta Academiae Medicinae Bengbu 24: 145-146.
- Song LY, Yan WS, Zhao T (2002) Detection of Mycobacterium tuberculosis in lung cancer tissue by indirect in situ nested PCR. Di Yi Jun Yi Da Xue Xue Bao 22: 992-993.
- Yesong WXQ , Lifa X (2004) A case report on pneumoconiotu- berculosis complicated with lung cancer and Mycobacterium tuberculosis- L form infection. Chin J Industrial Med.
- Zhang S, Guang-ling Z, Yan-sheng T (2009) Detection of Mycobacterium tuberculosis L forms infection in tissues of lung carcinoma. Chin J Public Health 25: 1317-1318.
- Yan-sheng T, Xing-kun C, Wei Z, Yan MA, Sheng-feng D, et al. (2013) Detection of Mycobacterium tuberculosis L-forms and MPB64 gene in breast cancer tissues. J of Practical Medicine 15: 2552-2555.
- Sheng TY, Kun CX, Tong H, Guang LH, Wei Z, et al. (2009) Study on the relationship between Mycobacterium tuberculosis L infection and lung cancer. Tumor 29: 1085-1089.
- Tian Y, Hao T, Cao B, Zhang W, Ma Y, et al. (2015) Clinical End-Points Associated with Mycobacterium tuberculosis and Lung Cancer: Implications into Host- Pathogen Interaction and Coevolution. Bio Med Research Intern p: 9.
- Alexander-Jackson E (1970) Ultraviolet spectrogramic microscope studies of Rous sarcoma virus cultured in cell-free medium. Ann N Y Acad Sci 174: 765-781.
- Van der Maaten M, Miller J (1976) Replication of bovine leukemia virus in monolayer cell cultures. Bibl Haemat 43: 360-362.
- Lysenko AP, Drogun AG, Shurinova (1998) Studies of influence atypical mycobacterial infection on AGID results with sera of cattle infected BLV (in Russian). Vet. nauka - proizvodstvu 33: 56-54.
- ShivRaj L, Patil SA, Girdhar A, Sengupta U, Desikan KV, et al. (1988) Antibodies to HIV-1 in sera from patients with mycobacterial infections. Int J Leprosy 56: 546-551.
- Lysenko AP, Vlasenko AP, Broxmeyer L (2014) Phenomenon of variability of mycobacteria and its use for detection of a tuberculosis infection.
- Lysenko AP, Vlasenko VV, Broxmeyer L, Lemish AP, Novik TP, et al. (2014) The tuberculin skin test: how safe is safe? The tuberculins contain unknown forms capable of reverting to cell-wall deficient mycobacteria. Clin Exp Med Sci 2: 55-73.
- Lysenko AP, Vlasenko VV, Lemish AP (2014) Detection of mycobacteria in tissues by means of the differentiating immunoperoxidase staining. Tuberculos i bolezni legkhih 10: 55-58.
- Duesberg PH (1987) Retroviruses as carcinogens and pathogens: expectations and reality. Cancer Res 47: 1199-220.
- Demochowski L, Grey CE (1957) Subcellular Structures of Possible Viral Origin in Some Mammalian Tumors. Ann NY Acad Sci : pp 559-615
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Dameshek W and Gunz (1965) Leukemia. Am J Med Sci 249: 115.
- Seibert FB, Feldmann PM, Davis RL, Richmond IS (1970) Morphological, Biological, and Immunological Studies on Isolates from Tumors and Leukemic Bloods. Ann NY Acad Sci 174: 690-728.
- Mankiewicz E (1965) Bacteriophages that lyse mycobacteria and corynebacteria, and show cytopathogenic effect on tissue cultures of renal cells of cercopithecus aethiops: a preliminary communication. Can Med Assoc J 92: 31-33.
- Seibert FB (1968) Pebbles on the hill of a scientist. St Petersburg Printing Company 1: 162.
- Dobrindt U, Reidl J (2000) Pathogenicity islands and phage conversion: evolutionary aspects of bacterial pathogenesis. Int J Med Microbiol 290: 519-27.
- Landman OE, Burchard WK, Angelety LH (1962) Lysogeny and bacteriophage adsorption in stable and reverting L-forms of Salmonella paratyphi B and Escherichia coli. Bacteriol Proc p: 53.
- Falagas ME, Kouranos VD, Athanassa Z, Kopterides P (2010) Review ÃÆâÃâââ¬Ãâââ¬Â¢Tuberculosis and malignancy. Q J Med 103: 461-487.
- Nelson EL, Pickett MJ (1951) The Recovery of L Forms of Brucella and their Relation to Brucella Phage. J Infect Dis 89: 226-32.
- Kruegar AP, Cohn T, Smith PN, McGuire CD (1948) J Gen Physiol 31: 477-488.
- Takahashi S (1979) L phase growth of Mycobacteria. 1. Cell wall deficient form of Mycobacteria. Kekkaku 54: 63-70.
- Broxmeyer L, Sosnowska D, Miltner E, Chacón O, Wagner D, et al. (2002) Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J Infect Dis 186: 1155-60.
- Devadoss PO, Klegerman ME, Groves MJ (1993) Phagocytosis of Mycobacterium bovis BCG organisms by murine S180 sarcoma cells. Cytobios 74: 49-58.
- Marcova N, Michailova L, Kussovsski V, Jordanova M (2008) Formation of persisting Cell Wall Deficient Forms M. bovis BCG during interaction with peritoneal macrophages in guinea pigs. Electronic J. of Biology 4: 1-10.
- Chauhan A, Madiraju MV, Fol M, Lofton H, Maloney E, et al. (2006) Mycobacterium tuberculosis Cells Growing in Macrophages Are Filamentous and Deficient in FtsZ Rings. J Bacteriol 188: 1856-1865
- Wagner PL, Waldor MK (2002) Bacteriophage Control of Bacterial Virulence. Infect Immun 70: 3985-3993.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences